Protocols
Protocols
A protocol is a detailed plan for your study. It includes a rationale for conducting the project, research question, inclusion/exclusion criteria, literature search plan, a method for data abstraction/data management and to evaluate the quality of studies.
Standards for protocols
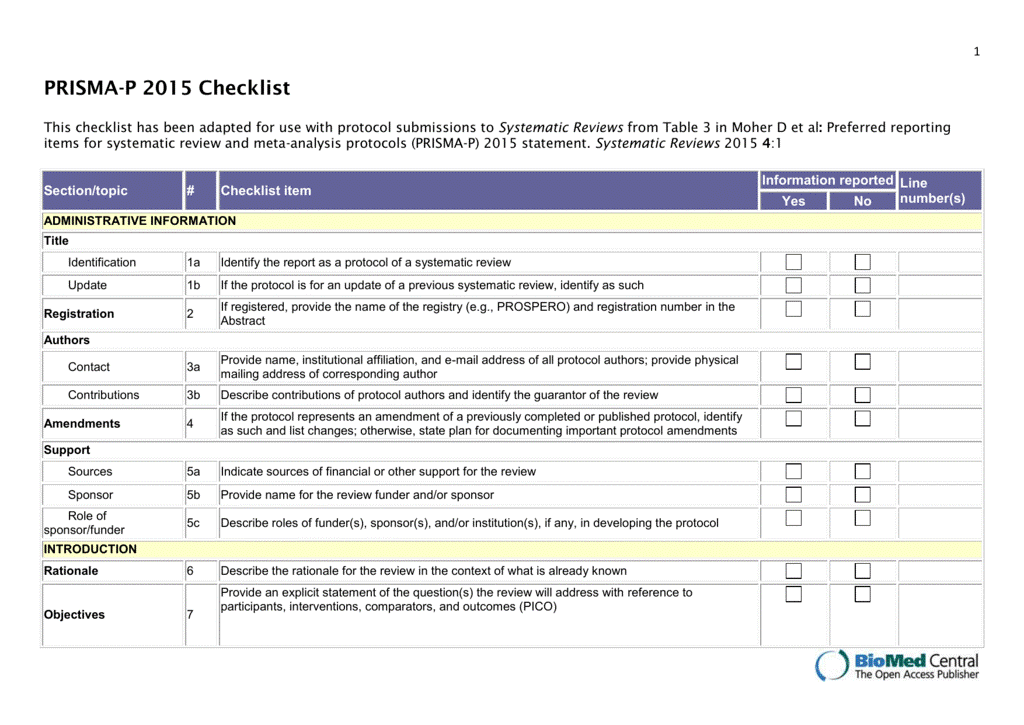
See Table 3 of the PRISMA-P 2015 Checklist for a list of the items to include in a systematic review protocol.
Protocol template
Link to a Protocol template based on PRISMA-P
Review Protocol Template by Sarah Visintini, licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Protocol registration
Registering your systematic review protocol promotes transparency, reduces the potential for bias, and helps to avoid duplication of reviews.
PLoS Medicine Editors. Best Practice in Systematic Reviews: The Importance of Protocols and Registration PLoS Med. 2011 Feb;8(2):e1001009.
PROSPERO International Prospective Register of Systematic Reviews
Publishing a procotol
The Cochrane Collaboration publishes protocols as does the journal Systematic Reviews
Many health care journals are publishing protocols now too, see for example the following protocols listed in PubMed.